By Karen Surabian, Contributing Writer
Patents provide a period of exclusivity and are a way to exclude others from making, using, or selling an inventor’s novel technology. For the National Institutes of Health (NIH), patents are an incentive for an outside party to license, develop, and commercialize NIH technologies that will benefit public health, especially those that require substantial further development by an outside party, such as therapeutics and diagnostics. Licensing these inventions to others willing to commercialize the technology fosters the development of the technology into a useful product.
The NIH Office of Technology Transfer (OTT) licenses technologies that are invented at NIH and works with the NCI Technology Transfer Center (TTC) to process patent applications on selected inventions. In addition, NCI TTC and NIH OTT work with outside partners using a variety of mechanisms to facilitate the commercial development of NIH technologies.
Mechanisms to access NIH materials for research or commercial development, or to partner with NIH in a collaborative project may include Material Transfer Agreements (MTAs), Confidential Disclosure Agreements (CDAs), Cooperative Research and Development Agreements (CRADAs), other types of collaborative agreements, and licenses (non-exclusive and exclusive).
Patents Issued in 2012
Anti-viral griffithsin compounds, compositions, and methods of use. Inventors: Barry O’Keefe,* Toshiyuki Mori,* and James McMahon* (US 8,088,729; issued January 3)
Nitric oxide–releasing diazeniumdiolated compounds. Inventors: Joseph Hrabie,* Frank DeRosa,* and Larry Keefer* (US 8,093,343; issued January 10)
Human monoclonal antibodies that specifically bind IGF-II. Inventors: Dimiter Dimitrov* and Yang Feng* (US 8,105,598; issued January 31)
Human immunodeficiency virus type 1 (HIV-1)-neutralizing human single-chain antibodies with improved breadth and potency. Inventors: Dimiter Dimitrov* and Mei-Yun Zhang* (US 8,110,192; issued February 7)
Methods of gene therapy using nucleic acid sequences for ATP-binding cassette transporter. Inventors: Rando Allikmets,* Kent Anderson, Michael Dean,* Mark Leppert,* Richard Lewis, Yixin Li, James Lupski, Jeremy Nathans, Amir Rattner, Noah Shroyer, Nanda Singh, Philip Smallwood, and Hui Sun (US 8,129,353; issued March 6)
In situ assembling of protein microarrays. Inventors: Deb Chatterjee,* Kalavathy Sitaraman,* James Hartley,* Cassio Baptista,* and David Munroe* (US 8,148,302; issued April 3)
Potent combinations of MRNA transport elements. Inventors: Barbara Felber,* Sergey Smulevitch,* and George Pavlakis* (US 8,163,542; issued April 24)
Inactivators of 06-alkylguanine-DNA alkytransferase. Inventors: Robert Moschel,* Matthew Karl Moschel (legal representative), Anthony Pegg, Sahar Javanmard,* Natalia Loktionova, and Gary Pauly* (US 8,188,055; issued May 29)
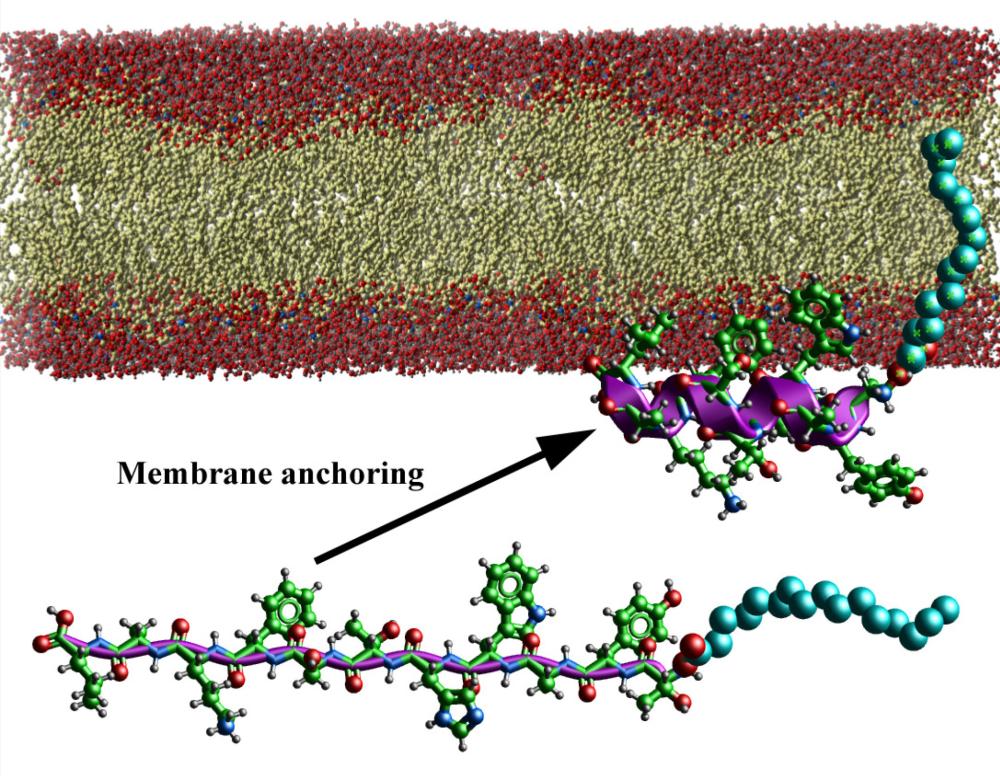
Smoothened polypeptides and methods of use. Inventors: Nadya Tarasova,* Michael Dean,* and Hong Lou* (US 8,198,402; issued June 12)
Inhibitor of DNA methylation. Inventors: Eric Selker, Cindy Matsen, Peter Jones, Jonathan Cheng, Sheldon Greer, and Victor Marquez* (US 8,207,142; issued June 26)
Method of treating pneumoconiosis with oligodeoxynecleotides. Inventors: Dennis Klinman* and Takashi Sato* (US 8,222,225; issued July 17)
HMGN polypeptides as immune enhancers and HMGN antagonists as immune suppressants. Inventors: De Yang,* Joost Oppenheim,* and Michael Bustin* (US 8,227,417; issued July 24)
Inhibitors of ubiquitin E1. Inventors: Allan Weissman,* Yili Yang,* and Jane Jensen* (US 8,242,160; issued August 14)
Fast electron paramagnetic resonance imaging (EPRI) in the CW EPR mode using rapid-scan in the presence of rotating gradients and direct detection with transmit/receive and data processing in a digital signal processing platform. Inventors: Sankaran Subramanian,# Nallathamby Devasahayam,# Janusz Koscielniak,* James Mitchell,# and Murali Krishna Cherukuri# (US 8,242,778; issued August 14)
Cellular and viral inactivation. Inventors: Yossef Raviv,* Mathias Viard,* and Robert Blumenthal* (US 8,268,602; issued September 18)
Fast electron paramagnetic resonance imaging (EPRI) using CW EPR spectrometer with sinusoidal rapid-scan and digital signal processing. Inventors: Sankaran Subramanian,# Nallathamby Devasahayam,# Janusz Koscielniak,* James Mitchell,# and Murali Krishna Cherukuri# (US 8,269,496; issued September 18)
Treating renal cancer using 4-[bis] 2-[(methylsulfonyl) oxy] ethyl] amino]-2-methyl-benzaldehyde. Inventors: Susan Mertins,* Susan Bates,# David Covell,* Geoffrey Patton,* Melinda Hollingshead,* and Rao Vishnuvajjalla# (US 8,273,797; issued September 25)
Inactivated influenza virus compositions. Yossef Raviv,* Mathias Viard,* Robert Blumenthal,* Robert Hogan, and Stephen Mark Tompkins (US 8,278,083; issued October 2)
Viral chemokine-antigen fusion proteins. Inventors: Larry Kwak* and Bira Arya* (US 8,318,177; issued November 27)
Marketing efforts for these technologies by NCI TTC and NIH OTT have led to many collaborations and licenses. For example, US Patent 8,198,402 (Smoothened polypeptides and methods of use; see figure) was licensed and is also part of a CRADA with a start-up company to commercially develop the technology. In addition, the technology was part of a nomination entitled “Novel Protein-Like Therapeutics for the Treatment of Cancer,” for which NCI researchers Nadya Tarasova, Ph.D., Synthetic Biologics and Drug Discovery Facility; Michael Dean, Ph.D., Laboratory of Experimental Immunology; and Sergei Tarasov, Ph.D., Structural Biophysics Laboratory; and former NCI researcher Hong Lou, Ph.D., received an award for excellence in technology transfer from the Federal Laboratory Consortium (FLC) Mid-Atlantic Region, and an honorable mention at the National 2011 FLC Awards.
*Denotes researchers who were with NCI at Frederick (NCI and SAIC-Frederick) at the time the patent was submitted.
#Denotes researchers who were with NCI Bethesda at the time the patent was submitted.
Special thanks to Mojdeh Bahar, J.D., M.A., CLP (chief, Cancer Branch, NIH OTT), Christopher Sappington (technology development administrative specialist, TTC, NCI), and Tere Diaz (technology development administrative specialist, TTC, NCI) for helping to compile the list of patents.
If You Are Not Sure, Ask
If you have any questions or are not sure which agreement is appropriate for your research situation, contact the NCI Technology Transfer Center office:
Telephone: 301-845-5465
Website: http://ttc.nci.nih.gov
Follow Us on Twitter
http://twitter.com/NCITechTransfer
Join our LinkedIn Group
http://www.linkedin.com/groups/NCITechTransfer-NCI-Technology-Transfer-Network-4688463/about