An anti-leukemia compound identified at NCI at Frederick is one step closer to entering clinical trials in humans thanks to a $4.2-million grant from the Cancer Prevention and Research Institute of Texas (CPRIT).
CPRIT recently gave the funds to Allterum Therapeutics, Inc., a Texas-based biotechnology startup company created to manufacture the compound, Allterum, and advance it to human trials. The grant represents the first step in the multi-million-dollar process to bring Allterum to the clinic.
Allterum is a monoclonal antibody that binds to interleukin 7 receptor, a protein on the surface of various cell types, including white blood cells known as T cells. The Cytokines and Immunity Section of the Cancer and Inflammation Program, part of the NCI Center for Cancer Research (CCR), discovered and studied the antibody before transferring it to Allterum Therapeutics for development.
According to Scott Durum, Ph.D., head of the Cytokines and Immunity Section and scientific advisor to Allterum Therapeutics, Allterum has shown promise in treating T-cell acute lymphoblastic leukemia (T-ALL) in his laboratory’s benchtop and mouse studies of cancer.
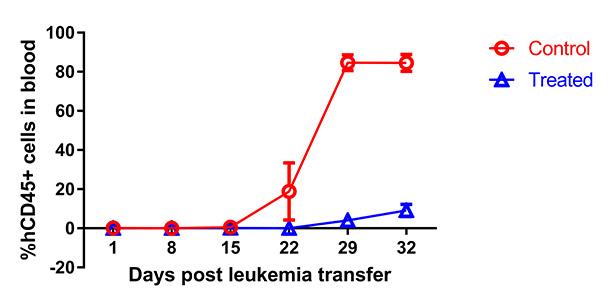
Mice with T-ALL that received Allterum lived significantly longer and had significantly fewer leukemia cells in their blood than mice treated with a control antibody. Allterum was also effective in mice that had relapsed after previous treatment with chemotherapy.
Despite the biological differences between mice and humans, the results were positive enough to suggest that Allterum may help to fill a critical gap in treatments available to leukemia patients, the majority of whom are children and adolescents. T-ALL accounts for 20 percent of leukemia cases diagnosed annually in the United States. Furthermore, one-fifth of pediatric T-ALL patients relapse despite chemotherapy, after which they have few treatment options and poor survival rates.
Durum’s group also found that Allterum may be less toxic than chemotherapy, which is often extremely harsh and puts children at risk of developing lifelong health problems. Similar to chemotherapy, Allterum is expected to weaken the immune system, but it shouldn’t create other toxic side effects. Children’s immune systems rebound quickly, meaning they should return to normal after the treatment ends.
Allterum Therapeutics plans to launch an early-stage clinical trial in 2020 in adolescents with relapsed leukemia. The initial efforts will be headquartered in Texas.
“We’re really happy,” Durum said. “Hopefully, we’ve contributed something.”
It Started with a Pot of Coffee
Allterum’s journey toward the clinic began five years ago while Durum was visiting Lisbon, Portugal. João Barata, Ph.D., an interleukin researcher and scientist at the University of Lisbon, learned that Durum, a fellow interleukin researcher, was in the city and invited him to a friendly chat over coffee.
During the conversation, Barata revealed that his laboratory had discovered mutations in the interleukin 7 receptor pathway, but he wasn’t sure if the mutations caused other biological effects. Durum offered to help, and Barata agreed, giving him copies of the mutated gene to bring back to the Cytokines and Immunity Section for further study.
Upon Durum’s return, Wenqing Li, Ph.D., a staff scientist in the section, inserted the genes into cells and performed several experiments that revealed the mutation’s role in driving T-ALL.
“And since then, we’ve continued to work on how it works,” Durum said. “But this also led us away from the basic science of it to try to do something about it.”
That initiated the search for a way to target interleukin 7 receptor. Julie Hixon, the section’s biologist, led those investigations, performed much of the work, and eventually discovered the antibody that would come to be known as Allterum.
“It’s been a great experience to be involved from the beginning stages and to see firsthand the development of a potential new therapeutic for pediatric leukemia,” Hixon said.
Other collaborators on the project included Sarah Cramer, Ph.D., former postdoctoral fellow, and Hila Winer, Ph.D., postdoctoral fellow, who investigated how the mutation cooperates with other biological functions to drive cancer. Scott Walsh, Ph.D., staff scientist, Chemical Biology Laboratory, CCR, produced the interleukin receptor protein used to generate antibodies and identified the site where Allterum binds to the mutated receptor. Two visiting Brazilian Ph.D. students, Priscila Zenatti and Gisele Rodriques, also assisted with the study.
“It’s an honor to think that a drug I helped develop could someday benefit a child who’s suffering from cancer,” Hixon said. “I’m grateful for the opportunity to participate in this project with top-notch collaborators.”