NCI recently announced the launch of the new National Cryo-Electron Microscopy Facility (NCEF) at the Frederick National Laboratory for Cancer Research (FNLCR). The launch comes while cryo-electron microscopy (cryo-EM) is enjoying the spotlight as a newly emerging, rapidly evolving technology with the potential to revolutionize the field of structural biology.
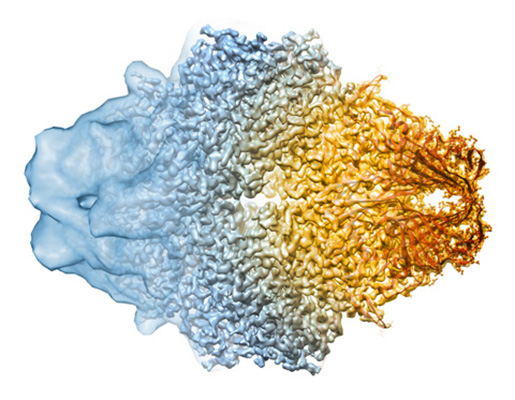
The last few months have seen an explosion of publications involving protein structures determined by cryo-EM single-particle analysis (a 3-D EM technique), many of them approaching atomic resolution. In 2013, there were only seven cryo-EM protein structures with resolutions better than 4Å (where one can begin to build models of the protein fold) deposited in the Electron Microscopy Data Bank (EMDB), a world-wide repository for EM structures. In 2015, 91 structures were deposited, and in the three months since the start of 2016, 45 additional structures at resolutions better than 4Å have been deposited into the EMDB.
This boom in structures is due to several factors: not only have there been breakthroughs in detector technology, which have allowed researchers to achieve a much better signal (having a high signal-to-noise ratio) from cryo-EM samples, but the techniques for analyzing data have also improved dramatically. Where once cryo-EM was a niche technique that was best used in conjunction with X-ray crystallography or nuclear magnetic resonance, it is suddenly in the spotlight, and researchers are increasingly looking for access to the high-end equipment necessary for data at very high resolution. The appeal: the potential of determining atomic resolution structures from very small amounts of protein, without first having to coax them to form a crystalline array, historically a first step for all structure determination efforts.
“There’s enormous interest in this technology, not just from present day practitioners of cryo-EM methods, but from a wide spectrum of biological scientists interested in protein structure,” said Sriram Subramaniam, director of the NCEF. “The prospect of directly visualizing how drugs bind, but without having to crystallize drug-protein complexes, is very exciting.”
The NCEF, which is slated to open for use by extramural researchers in the fall of 2016, will have the latest technology for data collection, including a new Titan Krios microscope, fitted with direct electron detectors for both high resolution and automated data collection.
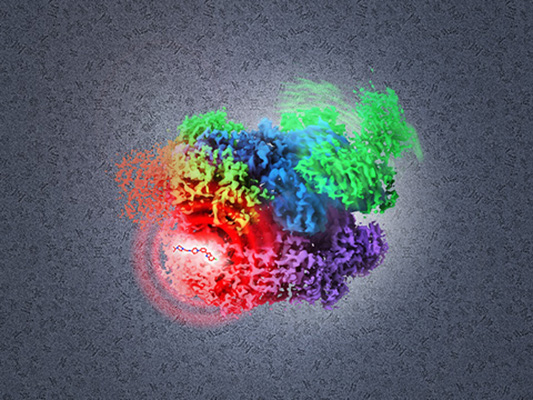
This facility is not FNLCR’s first foray into high resolution cryo-EM. In January 2015, in a collaboration between the Center for Cancer Research (CCR) and the FNLCR, an organization called the Center for Molecular Microscopy (CMM) was created at the Advanced Technology Research Facility (ATRF). In addition to high resolution cryo-EM, CMM (also directed by Subramaniam) specializes in 3-D cellular imaging with focused ion beam scanning electron microscopy, a technique that visualizes cellular ultrastructure at resolutions far surpassing that of even super-resolution light microscopy.
CMM is designed for two purposes. On the one hand, the CMM “collaboratory” works with CCR investigators, using 3-D EM techniques to delve into a variety of cancer research questions. On the other hand, in collaboration with scientists from the FNLCR and the RAS Initiative, CMM explores and implements new techniques and methods for high resolution 3-D imaging. These parallel tracks make it possible to undertake unique, cutting-edge science.
“At the CMM we don’t just look at one project in great depth, and we’re not a core facility either,” says Kedar Narayan, a CMM scientist who leads the cellular imaging projects. “Right now, we’re actively working with various labs to investigate very interesting biological questions; almost all of these projects also require significant technological innovation.”
Unlike traditional core facilities, CMM focuses on cutting-edge technology in 3-D EM and places it at the intersection between biology, imaging technology, and computation. CMM leverages new techniques for everything from labeling and sample preparation to data acquisition and analysis.
“The idea of having a high end lab doing a lot of technology development, but still having a lot of bandwidth for collaboration, is quite unique,” said Ulrich Baxa, a specialist in protein imaging at CMM.
CMM recently hosted a workshop for collaborators at the ATRF. In addition to reports on ongoing biological projects, there were presentations on novel methods for creating EM-compatible tags for proteins and RNA, automated correlation of light microscopy and EM data, as well as new methods for visualizing and reconstructing structures of membrane proteins and difficult RNA complexes using cryo-EM.
One of the biggest lessons learned in this first CMM workshop was the challenge, both for cellular imaging and for structural analysis with cryo-EM, of generating samples that really work with the technology.
For anyone interested in adding 3-D techniques to their research projects, “come and talk to us,” Narayan said. “These are really powerful techniques, but they have their limitations.” He added that knowing the ins and outs of these techniques is critical to finding the right experimental design and generating great data.
“People are really excited about cryo-EM recently, which is great of course,” Baxa said. “But they often don’t realize that the proteins still need to be at very high quality.”
And what brought the CMM about? “The creation of CMM is essentially a natural evolution in moving technologies newly developed in a specialist laboratory to a setting where they can begin to be used in unexpected and interesting ways by biologists who are looking for novel ways to complement what they already know how to do and may be completely different than what was envisioned by the developers,” Subramaniam said.
Lesley Earl is the scientific communications editor, Laboratory of Cell Biology, Biophysics Section, Center for Cancer Research.