A collaboration between the National Cancer Institute (NCI); the Frederick National Laboratory (FNL); and the National University of Ireland, Galway, has shown that two key biomarkers are co-expressed in an aggressive subtype of breast cancer and that inhibiting those two molecules can significantly improve patient survival outcomes.
The study, which was published in Proceedings of the National Academy of Sciences of the United States of America (PNAS), showed that the co-expression of the two inflammation-associated enzymes, nitric oxide synthase-2 (NOS2) and cyclooxygenase-2 (COX2), significantly reduced survival rates in patients with estrogen receptor-negative breast cancer.
The team, led by David Wink, Ph.D., principal investigator, Cancer and Inflammation Program, NCI at Frederick, also discovered that inhibiting COX2 and NOS2 with commercially available nonsteroidal anti-inflammatory drugs (NSAIDs) in combination with amino-guanidine significantly reduced tumor growth in a murine model of metastatic breast cancer.
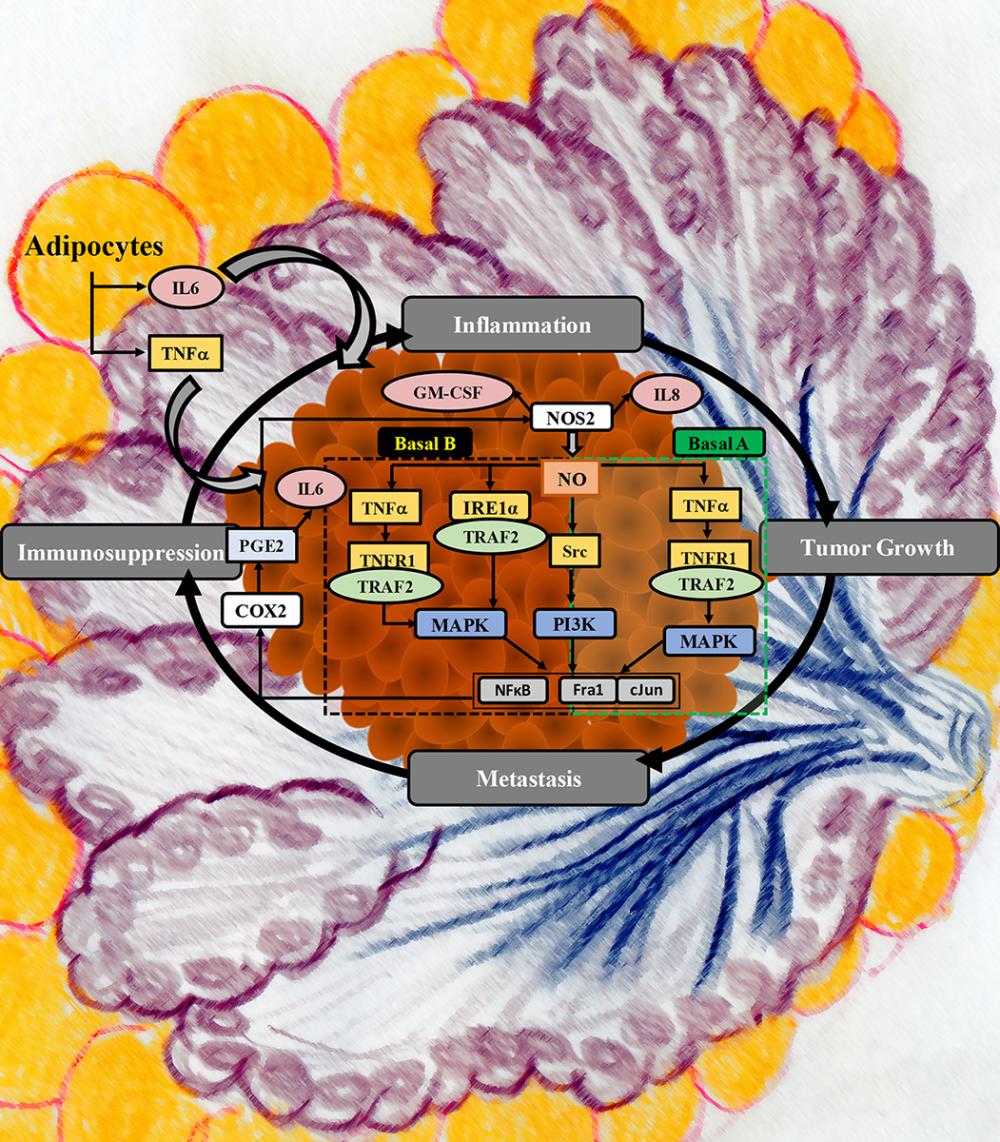
NOS2 and COX2 activate oncogenic pathways and produce two signaling molecules that have overlapping targets, which leads to a synergistic amplification of the tumor-promoting signal, according to Stefan Ambs, Ph.D., a key contributor to the study. Therefore, the team hypothesized that inhibiting NOS2 and COX2 might delay tumor growth and metastasis and perhaps lead to better responses from standard therapies.
“We showed that simultaneous inhibition of COX2 and NOS2 using commercially available inhibitors significantly reduced the primary tumor growth of MB-231-GFP xenografts in a murine model and were much more effective than either NOS2 or COX2 inhibition alone, thus demonstrating the beneficial effects of dual NOS2/COX2 therapy,” said Debashree Basudhar, Ph.D., a research fellow in the Cancer and Inflammation Program and the study’s lead author.
Furthermore, Basudhar and her colleagues believe their research can be broadened to other inflammatory molecules besides NOS2 and COX2, in part because both enzymes affect chemokine/cytokine signaling, immune response, and the cellular redox environment.
They are currently researching the role of other cell signaling molecules, known as eicosanoids, in controlling T-cell function in the tumor. They hope that understanding such intercellular communications will make it easier to predict how a tumor will respond to certain therapies.
The team’s study may have implications for future clinical use, as well, but they will have to do more extensive studies (some of which are currently underway) to determine whether there are any unfavorable outcomes associated with combined NOS and COX inhibitor therapy. According to Basudhar, an ongoing Phase II clinical trial originating from Dr. Jenny Chang’s group at Houston Methodist Hospital of the pan-NOS inhibitor NG-monmethyl-L-arginine (L-NMMA) with docetaxel in metastatic triple-negative breast cancer (NCT02834403) gives reason to believe that the addition of NSAIDs may be a viable course of action.
“Triple-negative breast cancer is highly metastatic and difficult to treat. Thus, it is really important to come up with new treatment options,” said Wink. “For us, it is thrilling to see a story come together that can have an impact in the clinic. This is one of the great things about being at NIH. It’s corny, but that’s why we come to work.”
Wink and Basudhar worked on the study with three researchers from the Optical Microscopy and Analysis Laboratory (OMAL) at FNL: David A. Scheiblin, William F. Heinz, and Stephen J. Lockett.
“OMAL has been essential in helping us understand the innate cellular communications between the cancer epithelium, the immune system, and the stroma,” said Basudhar. OMAL also helped facilitate a collaboration between Houston Methodist; the National University of Ireland, Galway; and Emory University to immunoscore the cancer stem cell niche.
For their part, Lockett, Heinz, and Scheiblin are developing a platform for 3D multiplex imaging of human and animal samples that will include laser-capture transcriptomics to interrogate specific regions of a tumor. They hope that a deeper understanding of these tumor niches will lead to potential new biomarkers and increasingly accurate models for therapeutic development.