Editor’s note: This article has been revised to give proper credit to the paper’s lead author, Tiara Byrd, Ph.D.
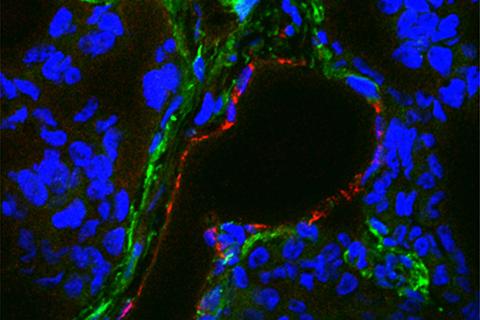
(Updated May 8) A study led by the Baylor College of Medicine and supported by NCI’s Center for Cancer Research (CCR) has demonstrated that chimeric antigen receptor (CAR) T-cell therapy can be used to treat solid triple-negative breast cancer (TNBC) tumors. The investigation is the first work using CAR T-cell therapy against TEM8, a cell surface protein that is frequently overexpressed both in TNBC cells and cells lining the blood vessels that sustain TNBC tumors.
After observing where and how TEM8 appears in TNBC tumors, the study’s authors genetically altered human T cells to express CARs that detect and bind to TEM8, causing the T cells to initiate an immune response against the TEM8-expressing cells.
The scientists believe that TEM8 presents an attractive target for CAR T-cell therapy because it is associated with aggressive cases of TNBC when present in tumors but exists only at low levels in healthy cells, which reduces the risk of unintended side effects. TEM8’s presence in both the tumor cells and the blood vessels sustaining those cells also allows the CAR T cells to attack the cancer on two fronts, increasing the therapy’s chance of success.
The team first compared the functions of second-generation and third-generation CAR T cells against TNBC tumors to evaluate which was more effective. Both types killed the tumor cells and inhibited the formation of mammospheres in organoid cultures, but the third-generation T cells stimulated a greater immune response against the tumor cells in vitro, remained longer in subjects’ bodies when tested in a live mouse tumor model, and contributed to higher survival rates in mice.
Based on those findings, the team studied the third-generation CAR T cells’ effects on TNBC tumors and patient survival in another mouse model. They found that the T cells significantly shrank the tumors by attacking both the TEM8-expressing tumor cells and the TEM8-expressing blood vessel cells, which destroyed the vessels in the tumors and starved the cancer cells of the blood necessary for their survival. As a result, the subjects’ median life expectancy nearly tripled. Mice with additional TNBC tumor models experienced similar benefits, with life expectancies that more than doubled after therapy.
Further tests revealed that the third-generation CAR T cells can block tumor metastasis and destroy existing metastasized tumors without causing significant damage to healthy cells and tissues, suggesting that the CAR T cells are safer and less toxic than chemotherapy.
Together, the team’s findings advance the research into CAR T-cell treatments against solid tumors—a concept that, until recently, had been labeled by many as nearly impossible due to a lack of promising targets. In fact, TEM8’s high frequency in other solid tumors, such as in the colon and lung, could make the team’s CAR T-cell therapy viable against those cancers, as well.
“This is one of reasons we think TEM8 may be a particularly valuable target,” said author Brad St. Croix, Ph.D., head of the Tumor Angiogenesis Unit in CCR’s Mouse Cancer Genetics Program and leader of the team that discovered TEM8 in 2000.
These results come at a significant juncture for CAR T-cell research. In 2017, the U.S. Food and Drug Administration approved the first two CAR T-cell therapies for clinical leukemia and lymphoma treatment. Growing interest in the technique, along with scientists’ questions about the limits of its effectiveness against solid tumors, may drive future research efforts with the potential to expand the results of this study and similar studies.
Along with St. Croix, CCR’s Christopher Szot, Ph.D., former post-doctoral fellow in the Tumor Angiogenesis Unit, and Steven Seaman, senior research assistant in St. Croix’s lab, made key contributions to the study.
Nabil Ahmed, M.D., led the large team of researchers from Baylor’s Department of Translational Biology and Molecular Medicine, while the study’s first author, Tiara Byrd, Ph.D., a postdoctoral fellow in the same department at Baylor, played a lead role in designing the study, developing the methodology, conducting experiments, and analyzing the data.
Other departments and centers at the Baylor College of Medicine, as well as researchers from Texas Children’s Hospital, Children’s Cancer Hospital Egypt, the University of British Columbia, Georg-Speyer-Haus, the University of Mainz, and the British Columbia Cancer Research Center, also contributed to the study.