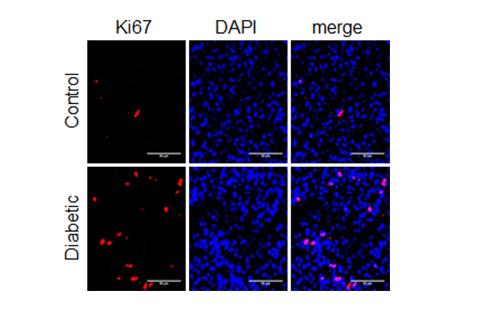
Scientists at NCI at Frederick, the Frederick National Laboratory for Cancer Research, and four Chinese institutions have uncovered why glioblastoma, the deadliest form of brain cancer, is more aggressive in diabetic patients than nondiabetic patients.
The team’s paper asserts that high amounts of a sugar called glucose trigger multiple biological processes that snowball to increase glioblastoma’s aggressiveness. Scientists had previously recognized a connection between glucose and aggressive glioblastoma, but until now, they didn’t know the underlying mechanism. The team hopes its discovery will facilitate studies of new potential treatments.
Most cancers, including glioblastoma, love sugars. Tumors gobble up glucose to sustain their growth and metastasis to new sites, but for glioblastoma in particular, the effects extend far beyond most tumors’ typical meals.
In those cells, excess glucose triggers an inflammatory response that causes two receptors, epidermal growth factor receptor (EGFR) and formyl peptide receptor 1 (FPR1), to be expressed in higher quantities on the cells’ surface. (Receptors are proteins that interact with other biological agents, called agonists, to receive signals that govern cell behavior.) The two molecular culprits cooperate to intensify tumor malignancy.
EGFR receives signals that control cell growth. It’s like a switch—when an agonist turns it “on,” it tells the glioblastoma cells to grow in number. Because high glucose makes it exist in greater amounts, it gets turned on more efficiently. The tumors become larger and harder to treat.
FPR1 is similar. It receives signals related to chemotaxis, the movement of cells from one location to another. A high-glucose environment makes the glioblastoma cells rapidly grow and invade, leading to a fast-spreading, highly aggressive cancer.
“Without high glucose, these receptors also mediate the malignant behavior of glioma cells, but not to the larger extent when you add diabetic-level glucose,” said Ji Ming Wang, M.D., Ph.D., the team’s senior investigator and the head of the Chemoattractant Receptor and Signal Section of the Cancer and Inflammation Program, part of the Center for Cancer Research laboratories at NCI at Frederick.
The team also found that high glucose compounds glioblastoma’s severity by stimulating the production of another protein called vascular endothelial growth factor (VEGF) in response to EGFR and FPR1 agonists. VEGF is critical for the formation of new blood vessels, which tumors require for survival and expansion. New blood vessels provide sustenance, make tumors more resilient, and allow cancer cells to invade surrounding tissues and metastasize by moving through the bloodstream.
“It’s a very typical combination of inflammation, cancer, and a very important metabolic disease, which is diabetes. So, it seems to be simplified, but actually, there’s a very close link between all these aspects,” Wang said.
The team verified the findings in both benchtop studies and in diabetic nude mice implanted with human glioblastoma cells.
Tests on Human Samples May Be Next
For Wang’s lab, the study is part of a long-running investigation into glioblastoma, inflammation, diabetes, and receptors. His group is particularly interested in G protein-coupled receptors, which include formyl peptide receptors like FPR1. Wang conceived the study after a visiting scientist demonstrated that high glucose increased another formyl peptide receptor in the human retina, causing blindness in some diabetic people.
“They are two different [receptors], but they have very similar properties. … And then my question was that, ‘If in diabetic patients the glioblastoma behaved so badly, what could high glucose do to tumor cells, in particular to their surface receptors that control cell migration and growth?’” he said.
With the answer now in hand, Wang and his collaborators hope to validate the findings by using samples from human patients. The eventual goal is a treatment that targets the tumors on multiple levels, including “turning off” the receptors exacerbating the malignancy.
A multi-pronged approach, which could include chemotherapy with several agents and a dietary regimen, may ultimately lead to better outcomes for patients. Studies in humans will be needed, however, to establish the effectiveness of such combination therapies.
“First of all, we need to control diabetes in patients. Secondly, we can control the tumor malignancy by using inhibitors of the receptors,” Wang said.
In China, scientists at the Third Military Medical University, the Maternal and Child Health Care Hospital of Shandong Province, the Peking University Health Science Center, and the Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine contributed to the study.
The teaser image on the Poster newsfeed depicts EGFR; adapted from an image by David Goodsell and published here under a CC BY 3.0 license.