Researchers in the National Cancer Institute’s Center for Cancer Research (CCR) and the Frederick National Laboratory’s Basic Science Program have discovered molecules that could keep the body from working against a cancer treatment.
The compounds prevent an enzyme called tyrosyl-DNA phosphodiesterase 1 (TDP1), a protein, from causing resistance to certain drugs. The researchers announced their work in a paper that was published in the Royal Society of Chemistry’s flagship journal Chemical Science and highlighted on the cover.
While TDP1 has necessary roles in normal cellular function, it can interfere with treatment by repairing the (intentional) damage to cancer cells caused by DNA topoisomerase 1 (TOP1) inhibitors, a class of cancer drugs.
TOP1 is an enzyme that sticks itself to cancer cells’ DNA and unknots coiled sections so that damage doesn’t occur when the cell divides. Because DNA damage can cause cells to die, TOP1 is a useful target for treatment. Some cancer treatments, such as camptothecin, bind to TOP1 and DNA, preventing the unknotting and creating damage that leads to cell death.
However, TDP1 can break this binding and remove the cancer drug, allowing the DNA repair to continue and making these treatments much less effective.
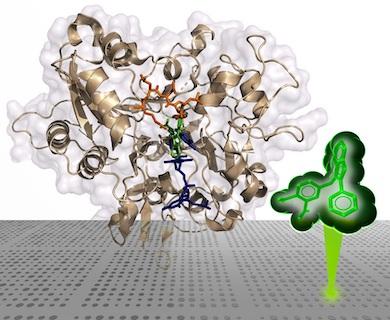
Structural Data Proves Molecules Inhibit TDP1
When the molecules that the scientists identified bind to a certain region of TDP1, they can prevent the enzyme from binding to and repairing the trapped TOP1-DNA complexes. So far, this has been tested just in the lab, but if it works in a person, it could be a useful therapy.
The group used a technique called small-molecule microarray to screen for molecules that bind to TDP1. They tagged TDP1 with a fluorescent label to make it visible in imaging and added it to glass slides printed with a library of 21,000 drug-like compounds. The compounds that bound to TDP1 were identified by the array dots on the slides that exhibited fluorescence under the microscope.
Even though the group’s plan was clear, they weren’t sure what they were going to find.
“I originally wanted to find some millimolar TDP1-binding fragments whose structural information might help us to design new TDP1 inhibitors using a fragment-based drug design approach. I didn’t anticipate getting any potent inhibitors. … Luckily, we found some compounds that show micromolar inhibition and bind at the TDP1 catalytic site directly,” said Xue Zhi Zhao, Ph.D., an associate scientist in the CCR’s Chemical Biology Laboratory.
Once the group found certain inhibitor molecules that bound to TDP1, they used a technique called X-ray crystallography to identify their structure when bound to TDP1.
“Basically, we take the crystals of the TDP1 protein soaked with the inhibitors and shoot X-rays at them. We can analyze the diffracted X-ray patterns through various computational methods, and it will give us the structure of the protein with the bound inhibitor,” said George Lountos, Ph.D., a scientist in Frederick National Laboratory’s Basic Science Program who works in support of the CCR Center for Structural Biology.
This technique’s advantage is its sensitivity. The researchers could see molecules that wouldn’t be picked up via other screens.
“And I have to add, we’re the only people, to my knowledge, in the world who are able to provide that type of data. … There are several international groups prolifically developing TDP1 inhibitors, but we have no idea whether their inhibitors are bound to TDP1 in relevant fashions. … There’s no structural data available for them,” said Terrence Burke, Ph.D., a senior investigator at the Chemical Biology Laboratory.
But now there is, thanks to the group. They have demonstrated structures of the inhibitor compounds bound to the protein.
“Luckily, we have a history of collaborating with Dr. Dave Waugh of the [Center for Structural Biology], who has successfully solved protein–ligand crystal structures for a number of our other molecular targets, and Dave played a key role in our current study,” Burke added.
Importantly, the researchers also learned how these inhibitors bind to TDP1. Several compounds they screened attach to a region known as the catalytic active site (the phosphate-binding pocket) and reach into both the DNA- and TOP1-binding regions.
This makes it possible to design inhibitors that can bind to multiple places, thus creating a stronger bind to TDP1, by modifying the compounds’ chemical structures. The researchers will use the information gleaned from the crystal structures to design new compounds.
Though the results are encouraging, more research must be done before these compounds can be developed any further. To protect their efforts, meanwhile, the group has filed a United States provisional patent with NCI’s support.
The group is optimistic about the discovery of an effective inhibitor. It could enable progress on a number of scientific fronts.
“A TDP1 inhibitor [could be] used as a chemical tool or a probe for biological systems. Because then you could study enzymes or the effect of the enzyme by using inhibitors. The second long-term [goal] is to potentially have something for in the clinic to overcome drug resistance in patients,” said Yves Pommier, Ph.D., a senior investigator in CCR’s Developmental Therapeutics Branch.
The Magic of Interdisciplinary Collaborations
The group credits their effective collaboration for the discovery. It simply wouldn’t have been possible otherwise. Fortunately, it’s not their first time working together.
“We have a long track record of efficient collaboration and trust,” said Pommier.
Their groups previously discovered certain HIV integrase inhibitors together, generating recently patented HIV therapeutics. Prior collaborations also focused on structure-based drug design efforts for other cancer and infectious disease drug targets.
“It was natural that we could join again in another research area which could be fruitful,” Pommier added.
The collaboration on the TDP1 study began when the Chemical Biology Laboratory took a retreat to the Catoctin Mountains. Zhao had a chance to review and discuss posters with other scientists, and one from the group of Jay Schneekloth, Ph.D., a senior investigator in the Chemical Biology Laboratory, caught his eye.
Zhao and Burke’s group partnered with the Developmental Therapeutics Branch, the Basic Science Program, and the Center for Structural Biology. They outlined the possibility of screening for TDP1 inhibitors with a large drug-like small-molecule library in the small-molecule microarray format Schneekloth’s laboratory had already developed to target RNA-binding molecules, and the collaboration unfolded from there. The project has also been supported by funding to Zhao from the NCI-CCR Staff Scientist/Staff Clinician Research Award program.
And it’s the kind of collaboration that could only happen at an institute like NCI.
“Why is the NIH the premier biomedical research center in the world? One of the reasons is the synergy that exists having more than 5,000 doctoral-level scientists crammed together with every discipline you can think of. There’s no necessity to write a grant proposal—you meet in the cafeteria, you discuss something, and within a day, you’re working on a project. What happens is collaborations start. It’s magic,” said Burke.